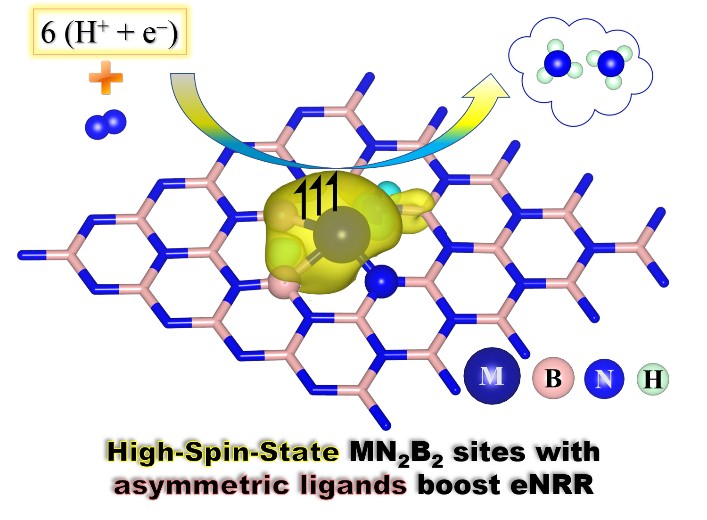
Single-atom catalysts (SACs) especially supported on two-dimensional nitrogen-doped carbon substrate have been widely reported to be able to effectively promote electrocatalytic N2 reduction reaction (eNRR). The precise design of single-metal-atom active site (SMAS) calls for fundamental understanding of its working mechanism for enhanced eNRR performance. Herein, by means of density functional theory calculations, we theoretically investigate the eNRR performance of nine prototypical SMAS, namely, MN2B2 (M: transition metals of IIIB, IVB and VB groups) which comprises of asymmetric ligands of N2B2 embedded in defective BN nanosheet. Our results reveal the significant role of spin state of SMAS in tuning the potential-determining steps of eNRR, in which MN2B2 site with higher spin magnetic moment (μ) is beneficial to reducing limiting potentials (UL) of eNRR. Specially, CrN2B2 (μ = 4μB), VN2B2 (μ=3μB) and MoN2B2 (μ=2μB) demonstrate high activity and selectivity to eNRR. The asymmetric ligands of N2B2 are deemed to be superior over mono-symmetric ligands. More importantly, our results demonstrate that breaking (or deviating) of the scaling relations between key N-containing intermediates (*N2H/*N2 and *NH2/*N2) on MN2B2 can be realized by enhancing spin state of SMAS which renders the active site a balanced N-affinity critical for efficient eNRR. This observation is validated by the calculated Sabatier volcano-shape relation between eNRR limiting potentials and N2 adsorption energy. Our study develops the guidance for catalyst design to boost eNRR performance by tuning the spin state of an active site.